Where Has 2D NMR Gone?
2D NMR is more than 30 years old (the original idea has been traced back to 1971). Initially developed during the 70s, it was brought to the masses at the end of the 80s. By then the newest spectrometers were already providing shortcut macros. With a single command it was possible, for the novice, to acquire, process and print a COSY, with another macro she could acquire an hetero-correlated experiment, etc.. Simplicity was necessary to attract the masses, but fashion was important too. Everybody experimented with 2D spectra, but they soon discovered it was too early. Computers were not fast enough. The proliferation of pulse sequences (most of which already forgotten) was confusing and neither phase sensitive spectra (unmanageable with slow computers and non-interactive software) nor magnitude-mode spectra (resolution-deficient and sensitivity-deficient at the same time) were satisfactory. The single exception was the COSY experiment: simple, robust, fast, informative, easy to run and easy to process. Its only possible drawback: it is out of fashion. Compare the COSY to a 1D spectrum: no need for phase correction. In most cases baseline correction and integration can also be skipped. Weighting is required, but you can always use a sine bell and give it no thought. In other words, not only COSY is the most useful and simple 2D experiment, it's also simpler than any 1D spectrum. Today's gradient-enhanced version is also as fast to acquire as a 1D spectrum. I don't mean that you must acquire it in all cases. Actually, it's an overkill in most cases. When only two multiplets share an identical coupling constant, it's obvious that they are the coupling partners of each other, you need neither the COSY nor the homo-decoupling. Most of the information is already available into the plain, 1D, proton spectrum. Let's say that the COSY is only required for 20% of your compounds. It's still a consistent number of 2D spectra, by the end of the year.
Look at this movie. The young chemist that you see at the beginning and at the end was born after 2D NMR. You'd expect she's at ease with it. The video is instead about a new product devoted to the semi-automated analysis of 1D spectra only. What happened? The guys at ACD Labs have recycled a few pieces of their code and created a new attractive product, called "1D NMR Assistant". It's encouraging to see new ideas in the field of NMR processing (although the ingredients aren't new, the overall concept is). There's another movie into Ryan's blog and others are coming. They save me the effort to actually try the program itself. I will cite a few sentences from Ryan's.
ACD/1D NMR Processor...can sometimes be viewed as bloated, and overly complex for a novice or non-expert user.
You are saying it! And you haven't even mentioned the 2D Processor! The 1D processor is already bloated by itself, according to you.
From the very beginning, we were developing a product with the NMR Spectroscopist in mind.
I can't buy it. From the very beginning the name of the company was "Advanced Chemistry Development", not "Advanced Spectroscopy Development". If you really had the spectroscopist in mind, why you removed 2D?
We have just de-emphasized some of those features in the software in an effort to greatly simplify the toolbars and interface. I believe that we reduced the learning curve significantly.
Admirable effort. It's a relief to hear somebody else recognizing what I am repeating from a lifetime: too many icons don't make an easier-to-use program.
The ultimate motivation behind this product was the simple idea that, "an NMR spectrum is a means to an end"
What's the end? Please don't conclude that the report is the end (the movie doesn't say such a thing). Anyway: the assistant can create the report from the spectrum even when the chemist understands the bare minimum.
There is nothing available that really helps users evaluate and interpret an NMR spectrum and it's relationship with a chemical structure. That is until now, IMHO. I think the development of ACD/1D NMR Assistant changes all that.
It changes that, but not all that. In the movie all the multiplets are isolated.
In my practice this is a rare circumstance, but evidently my compounds were more complicated than the average.
This product wasn't just built by a few software-oriented people within ACD/Labs.
We already know you are a chemist.
Over the next few weeks, I will be highlighting different features and workflows in the software to educate you on how it works and what it does.
This leaves me the burden to educate you on what it does not.
The assistant assumes that any multiplet can be analyzed regardless of the rest of the spectrum. It's the so-called "first order approximation". The lower the magnetic field, the less frequently this approximation can be applied. What happens in the remaining cases? If you recognize that there is strong coupling, either you resort to second-order analysis or you renounce to extract both the coupling constants and the chemical sifts (reporting a range for the latter). If you don't recognize it, the extracted parameters will not be accurate.
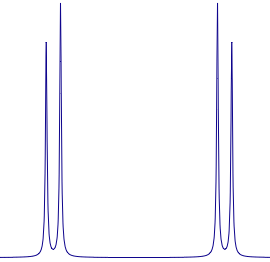 A spectrum subjected to strong coupling is not necessarily complicated. Look at this familiar AB pattern. Everybody knows the rule: the J corresponds to the splitting, while the chemical shift corresponds to center of mass of the doublet (not to the average position!).
A spectrum subjected to strong coupling is not necessarily complicated. Look at this familiar AB pattern. Everybody knows the rule: the J corresponds to the splitting, while the chemical shift corresponds to center of mass of the doublet (not to the average position!).Another celebrated case is represented by the ODCB. Here you really need to perform a generalized simulation, taking all signals into account. These cases are rare indeed, but if you are serious about extracting NMR parameters you can't avoid quantum mechanic forever.
 In one of the videos (the first one the blog, Nov 15) the assistant fails, at first, to extract the Js from a triplet of triplets. Then Ryan manually peak-picks two shoulders and feeds them to the program, which eventually succeeds. That's great, but can you always do without a line-fitting deconvolution? What's more, the example highlights the importance of the visual inspection. In the old days of CW NMR, the spectrum was plotted on a large sheet, similar to the A3 format (as we call it here in Europe). When I look at the ACD reports, where the actual plot is reduced to little more than a thumbnail, I wonder: "Do they observe them with a magnifying glass?". Of course the user is free to print magnified plots and this is not a terrible limitation, but what about the rendering on the screen? A few months ago Ryan admitted that his software was not as good-looking as the alternatives on the market, from which I concluded, erroneously, that the next version of his software would have filled the gap. No way, the graphics are still aliased, and, believe me, it was a little painful to see those movies. It brings you a decade back. When you get used to anti-aliased graphics, like in my case, you can't go back. Here is the aliased version of my previous ODCB picture.
In one of the videos (the first one the blog, Nov 15) the assistant fails, at first, to extract the Js from a triplet of triplets. Then Ryan manually peak-picks two shoulders and feeds them to the program, which eventually succeeds. That's great, but can you always do without a line-fitting deconvolution? What's more, the example highlights the importance of the visual inspection. In the old days of CW NMR, the spectrum was plotted on a large sheet, similar to the A3 format (as we call it here in Europe). When I look at the ACD reports, where the actual plot is reduced to little more than a thumbnail, I wonder: "Do they observe them with a magnifying glass?". Of course the user is free to print magnified plots and this is not a terrible limitation, but what about the rendering on the screen? A few months ago Ryan admitted that his software was not as good-looking as the alternatives on the market, from which I concluded, erroneously, that the next version of his software would have filled the gap. No way, the graphics are still aliased, and, believe me, it was a little painful to see those movies. It brings you a decade back. When you get used to anti-aliased graphics, like in my case, you can't go back. Here is the aliased version of my previous ODCB picture. It contains a good measure of shoulders, and they are all artifacts. While it's normally possible to discriminate authentic shoulders from fake ones like these, through a torture for your eyes, today we have eliminated the fake shoulders altogether. Anti-aliasing is implemented in hardware, not in software. Your graphic card is already capable to draw as shown in the first two examples. Those more advanced applications that draw better are doing nothing more than allowing the graphic cards to do their work. ACD Labs found the time to reassemble their old routines into a new attractive package but did not find the time to bring their drawing routines into the third millennium. What a pity!
It contains a good measure of shoulders, and they are all artifacts. While it's normally possible to discriminate authentic shoulders from fake ones like these, through a torture for your eyes, today we have eliminated the fake shoulders altogether. Anti-aliasing is implemented in hardware, not in software. Your graphic card is already capable to draw as shown in the first two examples. Those more advanced applications that draw better are doing nothing more than allowing the graphic cards to do their work. ACD Labs found the time to reassemble their old routines into a new attractive package but did not find the time to bring their drawing routines into the third millennium. What a pity!Do you know what else is missing? 2D spectroscopy. Maybe next year they'll be offering you the 2D NMR assistant...

1 Comments:
Having run 2 NMR facilities during my career I can comment that the majority of chemists do NOT run 2DNMR preferring instead to stick to 1D H1NMR in general. There has been a shift as more young people with 2D NMR interpretation skills have entered the workplace. Running the data is not the problem whether it's homonuclear or heteronuclear. In most modern facilities this is easy and push button. However, the NMR facilities themselves generally have to stimulate and train the walk up lab user base to run 2D in general.
The availability of 2D NMR tools is also not the issue. They have them, and they are well proven too (http://www.acdlabs.com/products/spec_lab/exp_spectra/2d_nmr/proc.html). But their software has been componentized in such a way as to offer the users their preferred options and capabilities. Some labs don't want to put 2D processing in the hands of their chemists. Rather they will provide static images as PDFs or printouts for interpretation. I'm not saying I agree with it but it is what it is.
I AGREE that more people should be using 2DNMR. I AGREE that they should have access to tools to workup data at the desktop.
Disclaimer: I'm an ex-employee of ACD/Labs and I can comment that they DO have SKILLED spectroscopists on staff. GOOD ONES. Check the literature and you'll see 12-15 peer-reviewed publications per year for the past couple of years. Science-based on NMR spectroscopy. Also, a company name is just that... a name. That one was chosen well over a decade ago before the company started working in spectroscopy. They also do Nomenclature, structure drawing and chromatography and....you get the point. Their role is to help chemists ADVANCE Chemistry. So, Advanced Chemistry Development is fine. How do you feel about Cambridgesoft as a name :-) or MestrecLab research ? Company names are just that...company names.
Post a Comment
<< Home